高度な分子プロファイルの基礎に向けた固形腫瘍のためのターゲット療法:
MyPathway( オープンラベル 第II相試験), 複数のバスケット研究
ペルツズマブ+トラスツズマブ、
エルロチニブ、
ベムラフェニブ、
ビスモードジブ
といった、分子標的薬のランダム化第II相試験(バスケット型研究)についての速報

http://ascopubs.org/doi/abs/10.1200/JCO.2017.75.3780
MyPathway( オープンラベル 第II相試験), 複数のバスケット研究
ペルツズマブ+トラスツズマブ、
エルロチニブ、
ベムラフェニブ、
ビスモードジブ
といった、分子標的薬のランダム化第II相試験(バスケット型研究)についての速報

http://ascopubs.org/doi/abs/10.1200/JCO.2017.75.3780
RAPID COMMUNICATIONS Clinical Trials
Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study
Purpose
Detection of specific molecular alterations in tumors guides the selection of effective targeted treatment of patients with several types of cancer.
These molecular alterations may occur in other tumor types for which the efficacy of targeted therapy remains unclear.
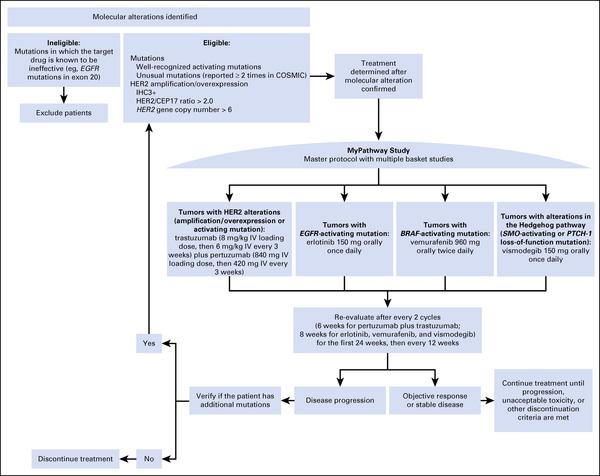
The MyPathway study evaluates the efficacy and safety of selected targeted therapies in tumor types that harbor relevant genetic alterations but are outside of current labeling for these treatments.
Methods
MyPathway (ClinicalTrials.gov identifier: NCT02091141) is a multicenter, nonrandomized, phase IIa multiple basket study.
Patients with advanced refractory solid tumors harboring molecular alterations in human epidermal growth factor receptor-2, epidermal growth factor receptor, v-raf murine sarcoma viral oncogene homolog B1, or the Hedgehog pathway are treated with pertuzumab plus trastuzumab, erlotinib, vemurafenib, or vismodegib, respectively.
The primary end point is investigator-assessed objective response rate within each tumor-pathway cohort.
Results
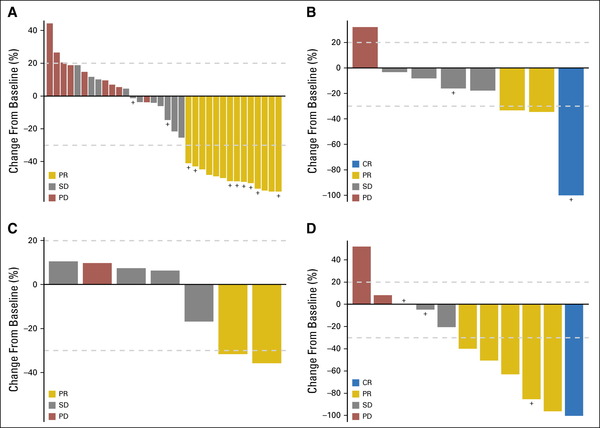
Between April 1, 2014 and November 1, 2016, 251 patients with 35 different tumor types received study treatment.
The efficacy population contains 230 treated patients who were evaluated for response or discontinued treatment before evaluation.
Fifty-two patients (23%) with 14 different tumor types had objective responses (complete, n = 4; partial, n = 48). Tumor-pathway cohorts with notable objective response rates included human epidermal growth factor receptor-2–amplified/overexpressing colorectal (38% [14 of 37]; 95% CI, 23% to 55%) and v-raf murine sarcoma viral oncogene homolog B1 V600-mutated non–small-cell lung cancer (43% [six of 14]; 95% CI, 18% to 71%).
Conclusion
The four currently approved targeted therapy regimens in the MyPathway study produced meaningful responses when administered without chemotherapy in several refractory solid tumor types not currently labeled for these agents.


